This new interview series includes a broad array of voices from the Africa CDC; CEPI; Gavi, the Vaccine Alliance; Moderna; Pfizer; and more on lessons learned leading the historic global effort to develop, distribute, and provide equitable access to COVID-19 vaccines and perspectives on the future of immunization.
The rapid development of the COVID-19 vaccines is having a profound effect on the world. This collection takes a closer look at the development, rollout and future of vaccines, including challenges that need to be overcome on the path to recovery.
FEATURED INSIGHTS
Article
Who’s left? Engaging the remaining hesitant consumers on COVID-19 vaccine adoption
-
While more Americans receive and show openness to receiving the COVID-19 vaccine, concerns persist among guardians of children...
Article
When will the COVID-19 pandemic end?
-
Europe and the United States have entered the endemic stage of the COVID-19 outbreak: the virus is widespread, is significantly...
Article
Not the last pandemic: Investing now to reimagine public-health systems
-
The COVID-19 crisis reminds us how underprepared the world was to detect and respond to emerging infectious diseases. Smart investments...
COVID-19 vaccines: The road to recovery and beyond
Collection
COVID-19 vaccine advances and takeaways for 2022
With billions of COVID-19 vaccine doses administered, the pandemic response has proved what collaborative science can do. But...
Interview
Africa’s plan for a continent-wide pandemic recovery
-
Dr. John Nkengasong, director of Africa Centres for Disease Control and Prevention (Africa CDC), explains what’s needed...
Interview
Finding solidarity in an effort to vaccinate the world
-
Dr. Seth Berkley, CEO of Gavi, the Vaccine Alliance, strengthens ties with partners to help increase global immunity in the context...
Meet some of our experts
The pandemic that is shaping us
As COVID-19 evolves, we asked some of our experts to share their perspectives on their experiences, the challenges that lie ahead, and the pandemic that is shaping us.
VACCINES AND THERAPEUTICS DEVELOPMENT
Article
Fast-forward: Will the speed of COVID-19 vaccine development reset industry norms?
-
A review of the funding, operational, technological, and regulatory factors that allowed for fast development of COVID-19 vaccines shows which will remain relevant for future efforts—and which won’t.
Article
Africa needs vaccines. What would it take to make them here?
-
For decades, private companies, investors, and public-health leaders have taken a pass on domestic vaccine manufacturing. But a confluence of circumstances may be changing the calculation.
Article
On pins and needles: Tracking COVID-19 vaccines and therapeutics
-
Key stakeholders will need to continue to monitor and adapt to new data and new variants emerging across the globe to respond effectively.
Article
Why tech transfer may be critical to beating COVID-19
-
How quickly COVID-19 vaccine production ramps up will depend on technology transfer—the capabilities and processes that can speed vaccines from development to manufacturing. Are we ready?
VACCINE ROLLOUT & IMMUNIZATION
Interview
Moderna’s path to vaccine innovation: A talk with CEO Stéphane Bancel
-
Moderna’s delivery of a COVID-19 vaccine comes on the heels of revolutionary science that may accelerate innovation across the industry well into the future.
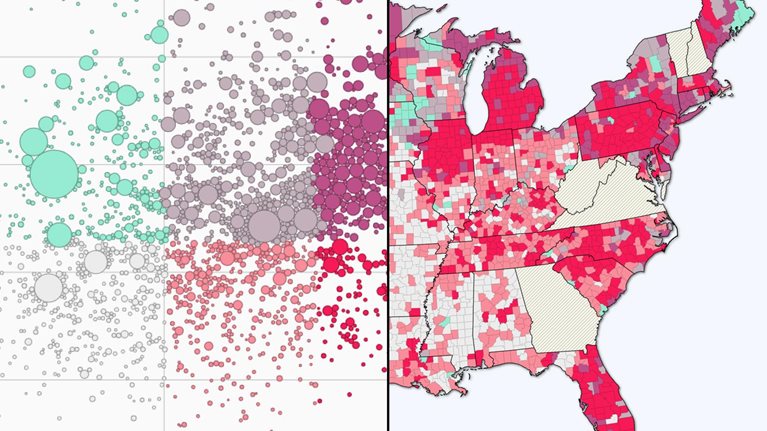
Interactive
COVID-19 vaccine distribution
Explore the US rollout of COVID-19 vaccinations in comparison to the impact of cases.
Article
A light at the end of the tunnel: US COVID-19 vaccine administration
-
More than a year after the COVID-19 pandemic began, a line of sight exists that promises enough vaccine for every eligible adult in the United States. This breakthrough offers hope, but also underscores an urgent need to plan the next phase of distribution and beyond.
Article
‘None are safe until all are safe’: COVID-19 vaccine rollout in low- and middle-income countries
-
Despite persistent supply issues, in-country delivery and demand for COVID-19 vaccines is likely to be the next challenge for LMICs.
Article
Getting to work: Employers’ role in COVID-19 vaccination
-
Employers can consider a set of actions that supports COVID-19-vaccine adoption among employees by building conviction and making vaccination as convenient and “costless” as possible.
Article
“Getting shots into arms”: How US states are addressing the vaccine distribution challenge
-
COVID-19 vaccine administration represents a significant challenge: States can accelerate progress through five lessons including establishing, enabling, and managing administration capacity.
Article
Is the world up to the challenge of mass COVID-19 vaccination?
-
Amid the unforeseen effects of the pandemic on at-risk and lower-income communities, governments and organizations around the world must convene to ensure effective distribution of newly developed vaccines.
Article
The risks and challenges of the global COVID-19-vaccine rollout
-
A realistic assessment of the heroic effort to administer billions of doses of COVID-19 vaccines to an agonized global population is necessary—the stakes could not be higher.
Vaccine Adoption
Article
Getting to work: Employers’ role in COVID-19 vaccination
-
Employers can consider a set of actions that supports COVID-19-vaccine adoption among employees by building conviction and making...
Article
COVID-19 vaccines meet 100 million uncertain Americans
-
More than 100 million Americans are uncertain about vaccination. Public- and private-sector leaders can take action to support...
Article
COVID-19 vaccine: Are US consumers ready?
-
While details of COVID-19 vaccine administration are still pending, healthcare stakeholders may want to consider their long-term...
Interactive
How Americans feel about COVID-19 vaccinations
Over half the respondents in the United States report an interest in taking a COVID-19 vaccine though safety and efficacy concerns...
Pandemic end and future preparedness
Article
Not the last pandemic: Investing now to reimagine public-health systems
-
The COVID-19 crisis reminds us how underprepared the world was to detect and respond to emerging infectious diseases. Smart investments...
Podcast
The COVID-19 vaccine: Lessons and challenges
-
The rapid deployment of vaccines is key in accelerating the return to normalcy.
Article
When will the COVID-19 pandemic end?
-
Europe and the United States have entered the endemic stage of the COVID-19 outbreak: the virus is widespread, is significantly...
Our Social Impact
Blog Post
For World Immunization Week, reflections on the fight against infectious disease
Our decades-long history in vaccine work, how we’re helping today, and what our vaccines leaders see going forward