Health systems around the world are under increasing strain because of the rising prevalence of chronic conditions, including diabetes, heart disease, and asthma. For more than 15 years, disease-management programs (DMPs) have been promoted as a solution to this problem. By carefully coordinating the delivery of high-quality care to patients with chronic conditions, the programs are supposed to enhance the patients’ health, reduce hospitalization rates, and lower treatment costs.
Unfortunately, initial experience with DMPs was often disappointing. Many of them produced, at best, only modest improvements in health outcomes, and few were able to decrease health care spending. Thus, many payor, provider, and health system executives have questioned whether the programs are worth their cost.
More recently, however, some DMPs have produced much better results. Germany’s diabetes program, for example, has reduced the incidence of some complications and has lowered the overall cost of care by 13 percent. Germany is also achieving good results with its programs for coronary artery disease (CAD) and chronic obstructive pulmonary disease (COPD). Several other countries have also begun to achieve good results with DMPs.
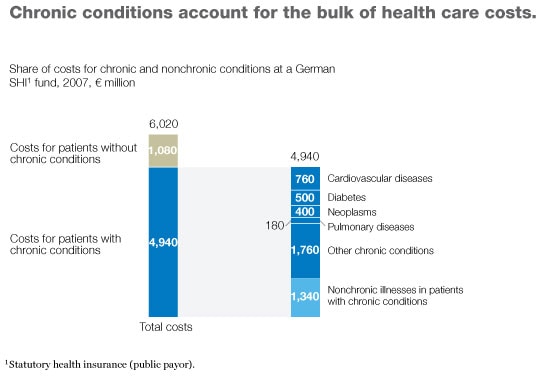
Why have some DMPs achieved some or all of their goals while others have failed? To answer this question, we analyzed successful and unsuccessful programs to identify the differences between them. The key, we discovered, lies in the programs’ design; five characteristics markedly increase the likelihood that a DMP can both improve health outcomes and lower costs. Based on our results, we have developed a checklist that payor, provider, and health system executives can use to determine whether a DMP they are considering or are already sponsoring is likely to be successful—and what they can do if they spot problems. In most developed countries, three-quarters or more of all health care spending is now devoted to patients with chronic conditions, and a large portion of that money is spent only on a small number of diseases (Exhibit 1). Thus, well-designed DMPs could do much to meet the current need to control health care spending while improving patient care.
Beginnings
The term “disease management” has often been used loosely to refer to general public-health campaigns (to promote regular exercise or influenza vaccination, for example), as well as to case-management programs tailored to individual patients. DMPs are neither of these. They are programs geared to specific groups of patients who all suffer from the same chronic condition. The patients receive a standardized, coordinated set of evidence-based interventions whose goals are to enhance the patients’ health and quality of life, reduce the need for hospitalization and other costly treatments, and thereby lower health care spending. Ideally, the savings obtained should exceed the programs’ cost.
The need for effective DMPs has been clear for at least two decades. Population aging, poor dietary choices, physical inactivity, tobacco use, and other factors have increased the prevalence of chronic conditions significantly. At the same time, the cost of care for patients with these conditions has risen markedly.
Widespread use of DMPs began in the United States in the 1990s as payors sought to rein in rising health care costs. By the end of that decade, DMPs for a wide range of different chronic conditions had been developed, and optimism ran high. Many DMPs were run by private companies, and some analysts predicted that DMPs would be a $20 billion industry by 2010.1
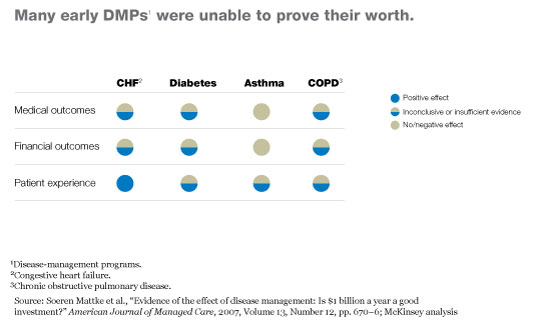
Unfortunately, once the results of these early programs were published, it became clear that many had not proved their worth. For example, an analysis of more than 300 early DMPs found that the programs did tend to improve quality of care, but in most cases there was no conclusive evidence that the improvements resulted in better long-term outcomes or significant cost savings (Exhibit 2).2 Only the heart-failure DMPs appeared to have increased patients’ satisfaction with treatment. Even worse, the asthma DMPs seemed to have had a negative impact on health outcomes and costs. Many other analyses of early DMPs from the United States and elsewhere produced similar results.
More recent experience
There remained room for hope, however. The authors of these analyses acknowledged that most of the DMPs they had studied had significant limitations: for example, the programs were too small to produce conclusive findings and did not always account sufficiently for the costs of the treatments being compared. Because of these limitations, the authors were not willing to conclude that DMPs could not work well. They simply had not been able to find evidence to prove the programs’ value.
Many health experts continued to believe that DMPs could be effective—a belief apparently vindicated by more recent results in a number of countries.
Germany’s DMPs
Almost a decade ago, Germany decided to reform the way it was funding its public payors, which provide health insurance coverage for about 90 percent of the population. One of its concerns was that some payors had been trying to attract only young, healthy patients, which left the other payors at an economic disadvantage. To discourage this type of cherry picking, Germany began giving its public payors extra funding for patients with chronic conditions. However, the size of the extra payments was capped to prevent health care costs from rising uncontrollably. Germany also coupled the increased funding with a requirement that the payors enroll patients with the most common chronic conditions in DMPs.3
To overcome some of the problems that had hampered earlier DMPs, Germany set minimum standards for them. For example, the programs’ clinical protocols had to be evidence-based, all care was to be coordinated by a single provider (a general practitioner, in most cases), and there had to be clear guidelines for when a referral to a specialist was warranted. In addition, the programs had to be approved by a federal health agency and then run nationwide.
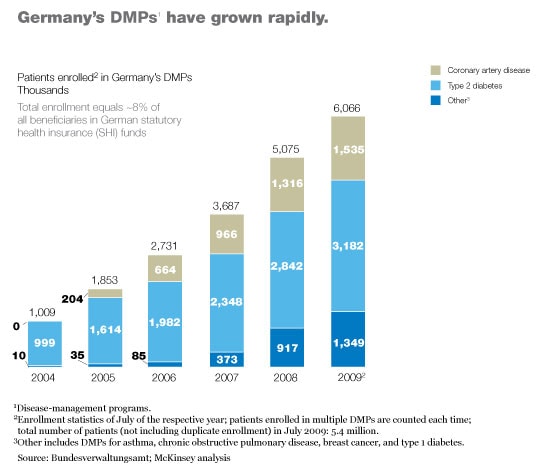
Germany’s type 2 diabetes program was the first to get off the ground. Although the program is only six years old, it has already enrolled more than three million patients (Exhibit 3) and has demonstrated that it markedly improves health care delivery to those patients. For example, the patients are now significantly more likely to have their feet checked regularly by a specialist, as a result of which the incidence of certain types of foot ulcer has plummeted.4 Preliminary evidence also suggests that the program may be decreasing mortality.5 Furthermore, patient satisfaction with treatment has risen markedly, and the overall cost of care has decreased; the small increases the program has produced in outpatient and pharmaceutical costs have been more than offset by a drop of more than 25 percent in inpatient costs (Exhibit 4).
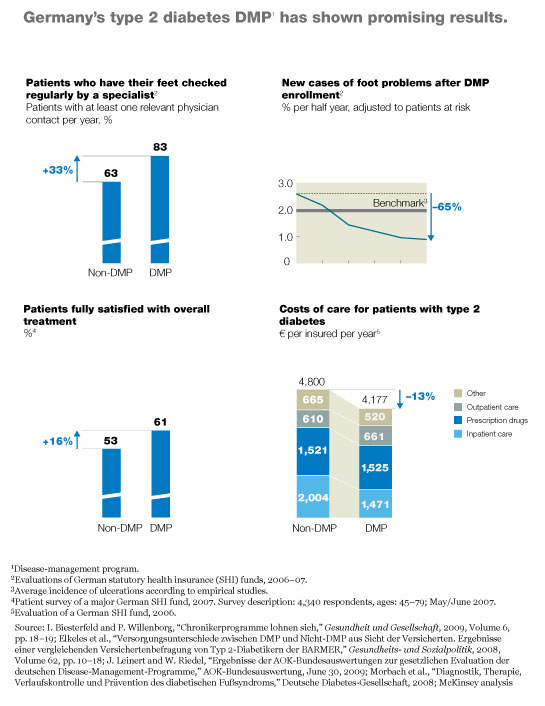
The program’s administrative costs average about €150 per patient per year, according to conversations we have had with large payors. Most of these costs result from additional fees paid to doctors. Because the payors’ average per-patient costs are slightly less than the extra funding they receive from the central fund (about €180 per beneficiary), they are able to capture all of the savings from the decrease in medical costs.
Germany’s other DMPs are newer, and their results have not yet been published. However, conversations we have had with several payors suggest that many of the programs, especially those for CAD and COPD, are also producing strong results.
Other recent experience
Other countries are also achieving success with DMPs, even though their programs do not have the nationwide scale that Germany’s programs have. In the Maastricht region of the Netherlands, for example, a DMP for asthma and COPD6 patients has increased treatment compliance by more than one-third and cut the hospitalization rate in half.7 It has also reduced total per-patient health care costs. Similarly, a DMP for COPD patients conducted by the US Veterans Health Administration has increased patients’ use of appropriate medications and lowered the rate of COPD-related hospitalizations and emergency-department visits.8
Japan’s Diabetes Prevention Program has also been quite successful. This DMP, which focuses on patients with impaired glucose tolerance (who are at high risk of diabetes), has helped the patients lose weight and reduced by two-thirds the risk that they would develop diabetes.9 In Italy, a DMP for heart-failure patients has reduced both the hospitalization rate and the duration of hospital stays and has lowered the cost of care per enrolled patient by about €980.10 A Swedish heart-failure DMP has also shortened the duration of hospital stays (although it has not lowered the hospitalization rate itself) and improved survival rates among enrolled patients.11 Sweden has also had success using DMPs for patients with hypertension and cancer. Many other successful programs from other countries are now being reported.
Drivers of success
We analyzed a wide range of DMPs from countries around the world to determine the characteristics that differentiate successful and unsuccessful programs. Five traits seemed to be the most important in ensuring that DMPs meet their goals: program size, simplicity of design, a focus on patients’ needs, the ability to collect data easily and analyze results, and the presence of incentives that encourage all stakeholders to comply with the program.
Size
Large DMPs are more likely to be successful than small programs because they benefit from economies of scale: resources can be pooled, and costs can be amortized across many patients. As a result, it is often easier to achieve net savings. Large programs are also more likely to improve their processes regularly, because they are usually better able to mine the data they are collecting and to use those analyses to refine their processes.
Furthermore, large DMPs often find it easier to develop and to ensure compliance with the evidence-based care pathways used for treatment. In small programs, individual physicians may feel that they have retained the right to manage patients according to their preference; the scale of large programs makes it easier to convince physicians to follow the care pathways and to make sure they have the equipment necessary for compliance.12
Small DMPs may also lack the statistical power to prove that better outcomes were achieved, particularly when the patients enrolled have high health care utilization rates. Many of the early DMPs had fewer than 100 patients in them; it is perhaps not surprising that their results were often inconclusive or negative.
In addition, small DMPs are more likely to be prone to selection bias. Both personal factors (age and severity of illness, for example) and population variables (including economic, geographic, and social differences) can influence a patient’s decision to enroll in a DMP, as well as health care utilization rates and therefore costs. Small programs have greater difficulties controlling for these factors, particularly if their eligibility criteria are so strict that only a minority of potential patients can be enrolled. In contrast, large DMPs with less strict eligibility requirements not only are more likely to achieve statistically significant results but also to produce results that can be generalized to all patients with a given disease. Many early DMPs were able to enroll less than 10 percent of their target populations; in some regions of Germany, participation rates are as high as 90 percent.
Simplicity
Many of the early DMPs were unnecessarily complex—they were not easy to run, their requirements were not easy to follow, and the bureaucratic hurdles they presented to physicians were high. It is perhaps not that surprising, therefore, that many of the programs had difficulty enrolling patients.
In contrast, successful DMPs tend to be very simple. For example, they avoid overly restrictive enrollment criteria, and they use uncomplicated care pathways that do not attempt to tailor treatment to multiple small subgroups of patients. This approach makes it easier for physicians to comply with the pathways and for the programs’ overseers to monitor what the physicians are doing.
In addition, responsibilities are clearly identified in successful DMPs. One person (often, a general practitioner) is responsible for all care delivered to a given patient (Exhibit 5). That person establishes a care plan with clear-cut goals, evaluates the patient regularly, and monitors how well the patient is adhering to the care plan. He or she is also responsible for coordinating any tests or treatments administered by specialists.
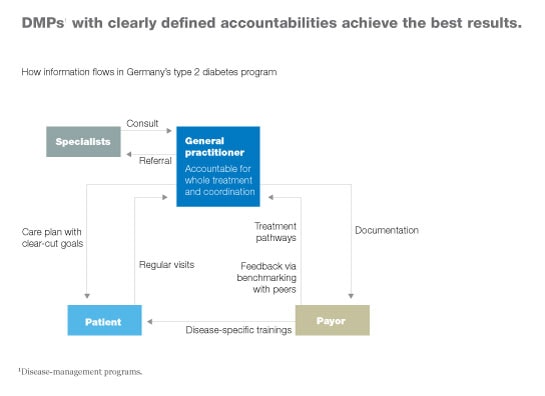
Simplicity of design also makes it easier to ensure that all stakeholders’ incentives are aligned appropriately—another characteristic of successful DMPs.
Patient focus
Many early DMPs failed because they did not take patients’ needs sufficiently into account. For example, one program we investigated was designed to study the use of a sophisticated telemonitoring device in patients with heart failure. However, two factors severely limited the program’s chances of success. First, the overwhelming majority of patients in the program had a relatively low probability of needing hospitalization or other costly interventions; as a result, it was impossible to achieve savings sufficient to offset the device’s high price. Second, many of the patients whose risk profiles were high enough to justify the device’s use were not technologically savvy and could not learn to operate it properly.
Successful DMPs focus on patients’ needs. The interventions they include are applicable to the vast majority of enrolled patients, as well as simple and easy to implement. The patients are given ongoing, disease-specific coaching to maximize their ability to care for themselves. In addition, the programs include regular physician visits and preventive health checkups so that the patients’ health status and compliance with treatment can be monitored. Because one person is responsible for coordinating each patient’s care, the patients are not subjected to duplicate treatments or other unnecessary inconveniences.
Finally, successful DMPs do not assume that patient compliance will be 100 percent at all times. To achieve as high a compliance rate as possible, the programs use patient segmentation approaches to develop targeted incentive schemes that encourage subgroups of patients to adhere to the programs’ protocols.
Information transparency
Many early DMPs did not have good mechanisms to prove their effectiveness because they did not have systems in place to monitor what patients were doing and what results were being achieved. In some cases, there were no clearly defined measures to gauge the programs’ success and no established methods for data collection. In other cases, data were collected, but there was often a long time lag before the data were processed. In addition, some of the early DMPs did not account fully for their own costs and thus could not determine whether they were actually saving money.
In contrast, successful DMPs define the metrics they want to measure about utilization rates, health outcomes (both short term and long term), patient satisfaction, and costs before they launch; they also put mechanisms in place to collect the necessary information from the start. They then collect data regularly and analyze it promptly.
Furthermore, the best DMPs use the collected data in two ways. First, the programs themselves evaluate the data routinely to assess their success and to determine whether refinements to their care pathways or other processes are required. Second, they send their data to authoritative third parties for independent analysis, review, and publication of results, because only independent evaluation guarantees that results are bias-free, and only by sharing data will the results be helpful to other programs. Use of the collected data in these two ways also gives patients greater confidence in the quality of care they are receiving, permits providers to benchmark their performance against their peers, and enables researchers to improve evidence-based care recommendations.
Incentives
Few of the early DMPs tried to ensure that all the stakeholders (physicians, patients, and payors) had an incentive to follow the DMP’s protocols. For example, many physicians object to having an outside party attempt to influence their treatment decisions, yet few early DMPs offered them incentives to conform to the program’s care pathways. In our experience, as long as one stakeholder lacks a strong incentive to move in the same direction as the others, a DMP is unlikely to produce good results.
Both nonfinancial and financial incentives can be used to align the interests of all stakeholders with the DMP’s protocols. For example, patients can be told that participation in the program can improve their quality of life, decrease the likelihood that their condition will worsen, and make it easier for them to navigate the health system. Where local practices permit, patients can be offered financial incentives, such as a decrease in copayment rates.
In a health system with competing payors, participation in a successful DMP can give a payor an image and marketing advantage. Participation also gives payors better access to patient-specific data and the cost savings that will accrue as the health status of enrolled patients improves. However, health systems can also offer payors more concrete incentives to participate; as discussed earlier, Germany gives payors a subsidy for each enrolled patient.
For physicians, participation in a successful DMP can help with patient retention. In addition, the regular training that good programs provide offers physicians an easy way to keep their medical knowledge up-to-date. Annual lump-sum payments can also be given to reimburse physicians for the added costs of care coordination and documentation.
Which incentives will work best may vary—from program to program, group to group, and even country to country. In countries that permit fee-for-service reimbursement, for example, physicians may have a financial stake in the treatments they currently deliver and may thus need especially strong financial incentives to comply with a program’s protocols. The key is to make sure that the incentives offered motivate all stakeholders to cooperate with one another and adhere to the care pathways.
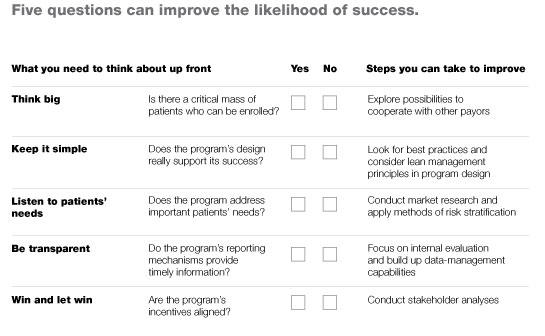
Any payor, provider, or health system that wants to gauge the likely success of a DMP it is considering, or check for problems in a DMP it is already sponsoring, should consult the checklist in Exhibit 6, which summarizes the points above. In addition to posing the questions that should be asked, the checklist outlines specific steps that can be taken to increase the odds of success.
Any group thinking of sponsoring a DMP must also realize that a large program takes years, not months, to get completely off the ground. It took Germany more than six years to fully implement its program for type 2 diabetes. During the first few years, the payors focused on motivating general practitioners to enroll appropriate patients and on explaining to those patients the benefits they could derive from the program. During this time, about one-third of appropriate patients were enrolled. Over the next two years, the payors built the infrastructure they would need to roll out the program at a larger scale; more patients entered the program as its capacity increased. From its earliest days, however, the payors collected and mined patient data, which enabled them to refine their care pathways and other processes. By the time the DMP was fully implemented, many of its processes were optimized, and all but a small percentage of appropriate patients were enrolled.
The success of this and other DMPs suggests that the programs, when designed in line with the criteria above, can reduce costs, increase quality of care, and improve health outcomes and patient satisfaction with treatment. We expect greater experience with DMPs to bring yet greater impact, perhaps by addressing the high rate of comorbidity in patients with chronic conditions.

